9th ICMRA Teleconference Minutes COVID-19 Real-World Evidence observational studies Working Group
Monday 11 September 2023
Chairs: Kelly Robinson (Health Canada - HC) and Peter Arlett (European Medicines Agency - EMA)
1. Welcome and introduction
Kelly Robinson and Peter Arlett opened this 9th ICMRA meeting on COVID-19 Real-World Evidence and Observational studies with a warm welcome to all the participants. The aim of the teleconference was to first reflect on what has been done and achieved over the last three years of collaboration, and second, to discuss five options put forward for the future of the Working Group (WG).
2. Technical WG Updates
a. COVID-19 Vaccine Pharmacovigilance Network (VPN led by MHRA + TGA)
Lessons learned during the COVID-19 pandemic with respect to pharmacovigilance were presented by MHRA and WHO during the VPN meeting in March 2023. Several topics of interest were identified and will be discussed at upcoming VPN meetings.
b. Pregnancy Observational Studies (led by EMA)
EMA provided an update on the CONSIGN project (Covid-19 infectiON and medicineS In preGNancy), launched in July 2020. The results show that pregnant individuals are unlikely to receive medicines, unless severely ill. If treated, the most used medications were antibiotics, anti-thrombotic agents, hydroxychloroquine and steroids during the early pandemic period. Important variabilities in medicines use across countries and healthcare settings were observed. The low numbers of exposures & adverse outcomes, coupled with a strong confounding by disease severity make it difficult to conclude on the impact of medicines used to treat COVID-19 during pregnancy on pregnancy and neonatal adverse outcomes. However, the fact that no teratogenic effect was identified during the study period tends to support vaccination of pregnant individuals to increase protection against the virus.
Two CONSIGN meta-analyses were performed under the umbrella of ICMRA with collaboration from CAMCCO Canada (3 regions), US Sentinel System, SET-NET (US CDC), George Washington meta-analysis and Saudi-FDA. These led to the generation of a goldmine of valuable data from 3 care settings, providing a unique perspective for regulators. The two final reports were received by EMA and are currently being assessed.
The main lesson learnt from the pandemic is that global collaboration is necessary for studying the impact of medicines in pregnancy. Leveraging and expanding the CONSIGN consortium’s expertise and experience beyond COVID will benefit pregnancies globally.
c. Building International Cohorts (led by HC)
Each regulator has been leveraging research teams and different data sources within their distinct jurisdiction to work on three main projects:
- Utilisation of steroids and COVID-19 patients (led by EMA)
- Natural history of coagulopathy (led by FDA)
- Validity and reliability of the COVID-19 case definitions (led by HC)
Numerous outputs thus far:
- Steroids (collaborative but independent analyses)
- Final EMA study report for steroid use published in EUPAS Register
- Results from Medicare and Sentinel System on corticosteroid use in non-hospitalised COVID-19 patients in JAMA
- CNODES is currently addressing HC’s comments on their final steroids report
- Coagulopathy (collaborative meta-analysis)
- Joint manuscript being finalised for submission to peer-reviewed journal
- COVID-19 Case Definitions (collaborative but independent analyses)
- EMA incorporated this topic into the steroids utilization project and final report
- FDA published their study using claims data in Pharmacoepidemiology and Drug Safety
- CNODES validation manuscript currently in press in CMAJ Open
3. Lessons learned and options for the WG moving forward
Feedback received on a survey on lessons learnt performed over February/March 2023 within the ICMRA WG led to the identification of 10 topics to be considered for the next steps. These were presented at the last WG meeting held in April 2023. Four regulatory agencies provided additional written comments over the summer. These 10 topics were then combined into 5 options for discussion by the participants to inform the future of the WG:
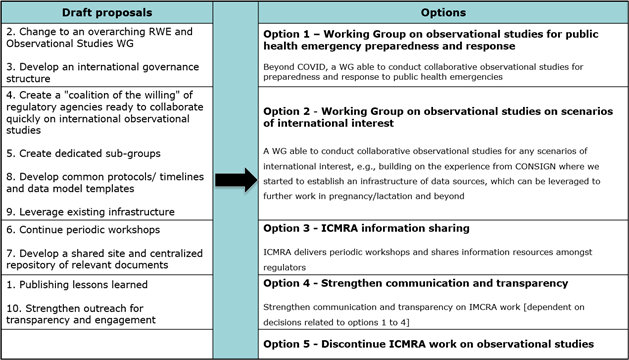
After the discussion, the WG members were invited to rank the options in their order of preference via a Slido poll (options not mutually exclusive). Thirty two participants out of thirty nine responded. The most supported was Option 2 (68%), followed by Option 1 (52%), with Options 3 (35%) and 4 (35%) being dependant of either Options 1 and 2. Option 5 was selected by only a hand full of members (6%), showing overall support for an ICMRA WG on observational studies.
A second question focused on the added value of collaboration on observational studies for the agencies (see image below). These results showed colleagues’ recognition of the importance of working together, of sharing information and of wide communication to ensure international preparedness and readiness for topics of interest.

4. Summary and next steps
Noting strong support for Option 2 but also strong support for Option 1, the Chairs thanked all participants for their active contribution, which provided substantial food for thoughts for the preparation of a new strategic vision on the future of the WG to be presented to the ICMRA leadership in November 2023.
Should colleagues be interested in getting involved in detailed discussions on the operationalisation of the ICMRA WG on observational studies, please email EMA and HC at kelly.plueschke[at]ema.europa.eu and rwe-dpmr[at]hc-sc.gc.ca .
Invited participants:
- Therapeutic Goods Administration (TGA), Australia;
- National Administration of Medicines, Food and Medical Technology (ANMAT) Argentina;
- National Health Surveillance Agency (ANVISA), Brazil;
- Health Products and Food Branch, Health Canada (HPFB-HC), Canada;
- National Medical Products Administration (NMPA), China;
- Center for the State Control of Medicines, Equipment and Medical Devices (CECMED) Cuba;
- European Medicines Agency (EMA), European Union;
- European Commission - Directorate General for Health and Food Safety (DG - SANTE), European Union;
- National Agency for Medicines and Health Products Safety (ANSM), France;
- Paul-Ehrlich-Institute (PEI), Germany;
- Health Product Regulatory Authority (HPRA), Ireland;
- Italian Medicines Agency (AIFA), Italy;
- Ministry of Health, Labour and Welfare (MHLW) and Pharmaceuticals and Medical Devices Agency (PMDA), Japan;
- Ministry of Food and Drug Safety (MFDS), Korea;
- Federal Commission for the Protection against Sanitary Risks (COFEPRIS), Mexico;
- Medicines Evaluation Board (MEB), Netherlands;
- Medsafe, Clinical Leadership, Protection & Regulation, Ministry of Health, New Zealand;
- National Agency for Food Drug Administration and Control (NAFDAC), Nigeria;
- Health Sciences Authority (HSA), Singapore;
- South African Health products Regulatory Agency (SAHPRA), South Africa;
- Medical Products Agency, Sweden;
- Swissmedic, Switzerland;
- Medicines and Healthcare Products Regulatory Agency (MHRA), United Kingdom;
- Food and Drug Administration (FDA), United States;
- World Health Organization (WHO);
- Austrian Agency for Health and Food Safety Ltd. (AGES), Austria;
- Danish Medicines Agency, Denmark;
- Office of Medical Technology, Health Information and Research (MTHIR), Israel;
- Office of Registration of Medicinal Products, Medical Devices and Biocidal Products (URPLWMiPB), Poland;
- Spanish Agency of Medicines and Medical Devices (AEMPS), Spain.

